Страница: 2/3
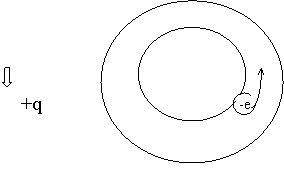
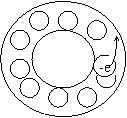
Roughly speaking, using the base cases of Born- Karman, let us consider a highly simplified case of one-dimensional conduction band. The first variant: a thin closed tube is completely filled with electrons but one. The diameter of the electron roughly equals the diameter of the tube. With such filling of the area at local movement of the electron an opposite movement of the ‘site’ of the electron, absent in the tube, is observed, i.e. movement of non-negative sighting. The second variant: there is one electron in the tube - movement of only one charge is possible - that of the electron with a negative charge. These two opposite variants show, that the sighting of carriers, determined according to the Hall coefficient, to some extent, must depend on the filling of the conduction band with electrons. Figure 1.
 | |||||
 | |||||
| | |||||
| |
а) б)
Figure 1. Schematic representation of the conduction band of two different metals. (scale is not observed).
a) - the first variant;
b) - the second variant.
The order of electron movement will also be affected by the structure of the conductivity zone, as well as by the temperature, admixtures and defects. Magnetic quasi-particles, magnons, will have an impact on magnetic materials.
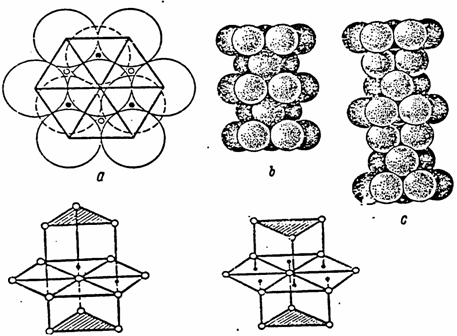
Since our reasoning is rough, we will further take into account only filling with electrons of the conductivity zone. Let us fill the conductivity zone with electrons in such a way that the external electrons of the atomic kernel affect the formation of a crystal lattice. Let us assume that after filling the conductivity zone, the number of the external electrons on the last shell of the atomic kernel is equal to the number of the neighbouring atoms (the coordination number) (5).
The coordination number for the volume-centered and face-centered densest packings are 12 and 18, whereas those for the body-centered lattice are 8 and 14 (3).
The below table is filled in compliance with the above judgements.
|
Element |
RH . 1010 (cubic metres /K) |
Z (number) |
Z kernel (number) |
Lattice type | |||||||||||||||||||||||||||||||||||||||||||||
|
Natrium |
Na |
-2,30 |
1 |
8 |
body-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Magnesium |
Mg |
-0,90 |
1 |
9 |
volume-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Aluminium Or |
Al |
-0,38 |
2 |
9 |
face-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Aluminium |
Al |
-0,38 |
1 |
12 |
face-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Potassium |
K |
-4,20 |
1 |
8 |
body-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Calcium |
Ca |
-1,78 |
1 |
9 |
face-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Calciom |
Ca |
T=737K |
2 |
8 |
body-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Scandium Or |
Sc |
-0,67 |
2 |
9 |
volume-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Scandium |
Sc |
-0,67 |
1 |
18 |
volume-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Titanium |
Ti |
-2,40 |
1 |
9 |
volume-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Titanium |
Ti |
-2,40 |
3 |
9 |
volume-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Titanium |
Ti |
T=1158K |
4 |
8 |
body-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Vanadium |
V |
+0,76 |
5 |
8 |
body-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Chromium |
Cr |
+3,63 |
6 |
8 |
body-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Iron or |
Fe |
+8,00 |
8 |
8 |
body-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Iron |
Fe |
+8,00 |
2 |
14 |
body-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Iron or |
Fe |
Т=1189K |
7 |
9 |
face-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Iron |
Fe |
Т=1189K |
4 |
12 |
face-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Cobalt or |
Co |
+3,60 |
8 |
9 |
volume-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Cobalt |
Co |
+3,60 |
5 |
12 |
volume-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Nickel |
Ni |
-0,60 |
1 |
9 |
face-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Copper or |
Cu |
-0,52 |
1 |
18 |
face-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Copper |
Cu |
-0,52 |
2 |
9 |
face-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Zink or |
Zn |
+0,90 |
2 |
18 |
volume-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Zink |
Zn |
+0,90 |
3 |
9 |
volume-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Rubidium |
Rb |
-5,90 |
1 |
8 |
body-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Itrium |
Y |
-1,25 |
2 |
9 |
volume-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Zirconium or |
Zr |
+0,21 |
3 |
9 |
volume-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Zirconium |
Zr |
Т=1135К |
4 |
8 |
body-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Niobium |
Nb |
+0,72 |
5 |
8 |
body-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Molybde-num |
Mo |
+1,91 |
6 |
8 |
body-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Ruthenium |
Ru |
+22 |
7 |
9 |
volume-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Rhodium Or |
Rh |
+0,48 |
5 |
12 |
face-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Rhodium |
Rh |
+0,48 |
8 |
9 |
face-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Palladium |
Pd |
-6,80 |
1 |
9 |
face-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Silver or |
Ag |
-0,90 |
1 |
18 |
face-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Silver |
Ag |
-0,90 |
2 |
9 |
face-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Cadmium or |
Cd |
+0,67 |
2 |
18 |
volume-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Cadmium |
Cd |
+0,67 |
3 |
9 |
volume-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Caesium |
Cs |
-7,80 |
1 |
8 |
body-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Lanthanum |
La |
-0,80 |
2 |
9 |
volume-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Cerium or |
Ce |
+1,92 |
3 |
9 |
face-centered | ||||||||||||||||||||||||||||||||||||||||||||
|
Element |
Z |
rs/ao |
theoretical |
calculated |
|
Cs |
1 |
5.62 |
1.54 |
1.43 |
|
Cu |
1 |
2.67 |
63.8 |
134.3 |
|
Ag |
1 |
3.02 |
34.5 |
99.9 |
|
Al |
3 |
2.07 |
228 |
76.0 |
Table 2. Calculation according to the models considered in this paper
|
Element |
Z |
rs/ao |
theoretical |
calculated |
|
Cs |
1 |
5.62 |
1.54 |
1.43 |
|
Cu |
2 |
2.12 |
202.3 |
134.3 |
|
Ag |
2 |
2.39 |
111.0 |
99.9 |
|
Al |
2 |
2.40 |
108.6 |
76.0 |
Of course, the pres
Реферат опубликован: 23/06/2009